Vectans Pharma is delighted to collaborate withBerlin Chemie expanding the international scope of Sitavig® to 30 additional countries.
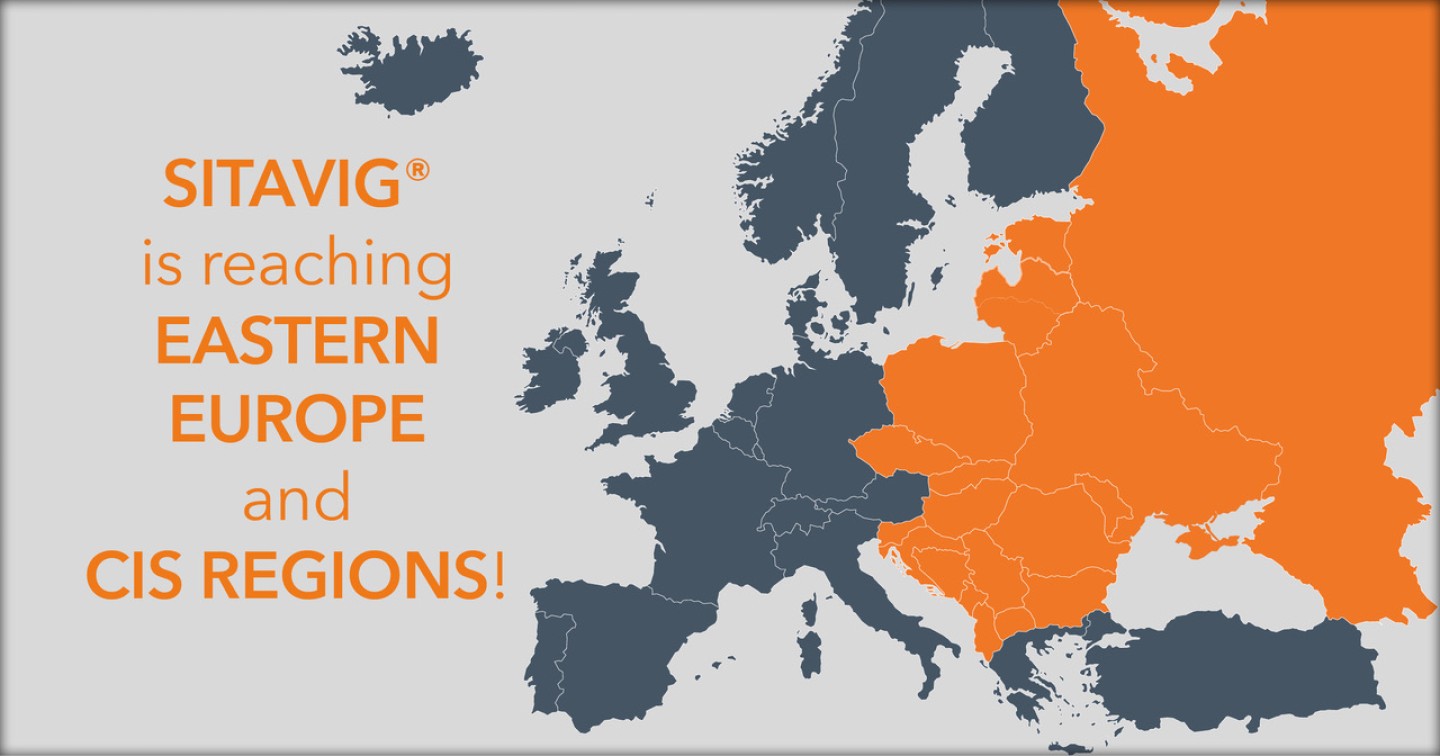

Vectans Pharma is delighted to collaborate withBerlin Chemie expanding the international scope of Sitavig® to 30 additional countries.

We are thrilled to welcome Medine Kockan as our new intern in Marketing and Strategic Innovation!
She will contribute to the many projects of our team. We are excited for her to participate in our continued growth!

Vectans Pharma will be present at the CPhI worldwide in Milan from November 9 to 11, 2021.

Vectans pharma is proud to announce that its one-per-episode Lauriad® aciclovir treatment tablet for herpes labialis, Sitavig® 50mg, has received the approbation of the ANSM for OTC commercialization in France. Issued from Lauriad technology, SITAVIG ensures an optimum and efficient localized concentration of acyclovir directly at the disease site.
A big step for Vectans , but also for the many patients in France prone to recurrent

Because changing the way medicines are delivered can improve patient’s life drastically. With Lauriad® we are committed to optimizing the existing therapeutic offer, bringing out new « best-in-disease » drugs.

Vectans Pharma is glad to inform you that BfArM granted a 3-year exemption of the sunset clause for LORAMYC® 50 mg, a muco‐adhesive buccal tablets issued from the Lauriad Technology, an innovative and patented drug delivery system ensuring targeted diffusion of Miconazole. In addition to Germany, LORAMYC® is also registered in other European countries such as the United Kingdom, France and Italy. LORAMYC® also benefits from an international presence through international licensing agreements in the United States and Asian countries.
Copyright © VECTANS PHARMA